Musculoskeletal Imaging / NGF-Inhibitor
NGF-Inhibitor
Imaging in Nerve Growth Factor Inhibitors (a-NGF)
Recently monoclonal antibodies that bind and inhibit nerve growth factor (a-NGF) have been introduced for treatment of osteoarthritis (OA) symptoms and have shown good analgesic efficacy and improvement in function in patients with OA.
Despite initial promising data, trials in OA had been suspended in 2010 by the Federal Food and Drug Administration (FDA) due to concerns over accelerated rates of OA progression (i.e. rapid progressive OA) to total joint replacement particularly in the large joints of the lower limb.
Since a-NGF therapies offer potential as the first new class of analgesics for many years, future studies of a-NGF compounds will require rigorous safety criteria. Imaging will play a crucial role in future clinical trials to define eligibility of potential participants and to monitor safety during the course of these studies and BICL has been involved in aNGF development since 2011 and is the leader in imaging of aNGF trials.
Imaging assessment will require baseline and frequent follow-up radiographs of both, the index joints and other large weight bearing joints to identify subjects at risk for rapid progressive OA (RPOA) and identify subjects on study with adverse events such as RPOA Type I or II so treatment can be discontinued. Additional magnetic resonance imaging examinations will be important during the course of a study in cases of unexpected joint pain or swelling or in cases of discrepancy between clinical symptoms, mainly pain and radiographic findings.
In the planning phase of clinical trials it is important to acknowledge that the role of radiologic assessment differs profoundly in efficacy and a-NGF studies. While in OA efficacy studies image acquisition and evaluation is optimized for sensitivity to detect minor changes between treated and non-treated subjects over time, in a-NGF studies the focus is on early detection of imaging findings that either put a subject at increased risk for an adverse outcome (eligibility) or may result in withdrawal from treatment (safety). These include but are not limited to RPOA and osteonecrosis
BICL has provided leading expertise to Pfizer’s Tanezumab program in developing the imaging program to monitor participants during the course of all aNGF studies for safety and eligibility since 2012.
A recent invitation by the Editors of the leading journal in OA research, Osteoarthritis and Cartilage, to BICL’s Drs. Roemer and Guermazi to be Guest Editors for a Special Issue on Imaging in aNGF trials, which was published in January 2015, reflects the highly dedicated involvement of BICL in aNGF development.
Eligibility
Imaging is paramount for the eligbility definition of participants into aNGF clinical trials. Diagnoses of exclusion for eligibility are those that potentially increase the risk of rapid progressive osteoarthritis (RPOA) Type I or II. These diagnoses include pre-existing atrophic OA and RPOA, subchondral insufficiency fractures and potentially severe malalignment of the knee. Additional diagnoses of exclusion for eligibility may be chondrocalcinosis, other arthropathies, e.g. rheumatoid arthritis, gout, systemic metabolic bone or joint diseases and also include primary or metastatic malignant tumors. In addition, fractures (stress, traumatic, pathologic) detected by either radiography or MRI are reason to exclude a subject from entering a trial regardless of cause of fracture.
Safety
Diagnoses relevant for safety after enrolment (i.e. joint safety findings) are rapid progressive osteoarthritis (RPOA) Types I and II, subchondral insufficiency fractures, osteonecrosis and pathologic fractures plus incidental findings of these entities described. Several of these diagnoses have non-specific findings on the radiograph or cannot be detected radiographically in early stages. Thus, in cases of inconclusive or suspicious radiography an additional MRI examination will commonly be acquired to rule out or confirm some of these diagnoses especially in early stages and thus, MRI findings need to be considered in addition.
A common image acquisition process is that potential participants will be screened by radiography for eligibility criteria, using a conservative approach to allow subjects being included into the study. Once included into a study, radiography will be the first line imaging approach to monitor safety, but MRI scans of any or all joints may be requested if needed (i.e. “for cause”, commonly requested due to equivocal radiographic findings or discrepancies between radiography and clinical symptoms).
Role of X-Ray and MRI
The radiographic imaging acquisition protocol that is being recommended for the knee in a-NGF studies is the posterior-anterior view in a modified Lyon-Schuss technique. This technique has been shown to provide the best precision for evaluating joint space narrowing in clinical trials.
The MRI protocol should be a standard clinical protocol or an abbreviated protocol consisting of at least a sagittal and coronal proton density or T2-weighted fat suppressed sequence and a coronal or sagittal T1-weighted non-fat suppressed sequence. The use of 1.5 T or 3 T large bore MRI is recommended for assessment of joints in aNGF studies. 1.0 T or 1.5 T extremity scanners may be used alternatively but are only applicable to image the knee joint and not other joints commonly assessed in aNGF studies such as the hip or shoulder.
Illustrative Cases
-

DMOAD Efficacy Study
Joint space narrowing (JSN) progression in efficacy study. Baseline radiograph shows definite medial JSN consistent with osteoarthritis Kellgren-Lawrence grade 3 (arrows). No relevant osteophytes are observed. Finding consistent with atrophic OA. 12 months follow-up image shows definite progression with increase in JSN medially (arrows). Patient will be staying on study despite the drug not being efficacious in regard to slowing of structural progression.
“Drug not efficacious, but patient stays on study.”
-

aNGF Study
Figure 2. Same example as Figure 1. In aNGF trials atrophic appearance of baseline image would result in exclusion at eligibility. The 1-year follow up images qualifies as rapid progression according to study-specific pre-defined cut-offs. As a result subject would be withdrawn from study for safety reasons.
“RPOA Type I - safety event; patient withdrawn from study.”
-

aNGF Study
Figure 3. aNGF study. Baseline image shows definite osteoarthritis Kellgren-Lawrence grade 3 with presence of osteophytes and medial JSN. At 12-month follow-up severe disintegration of the medial compartment including collapse of the tibial plateau is obserserved, which results in withdrawal of subject from study.
“RPOA Type II - safety event; patient withdrawn from study.”
Potential Adverse Events in aNGF Studies
-

Figure 1
Figure 1. Rapid progressive osteoarthritis Type I (RPOA Type I) over the course of 18 months. A. Baseline radiograph shows normal medial and lateral joint space width. B. 6 months follow-up X-ray depicts definite medial joint space narrowing (arrows) with persistent absence of osteophytes medially. C. Radiograph acquired 8 months after image B shows progressive loss of joint space medially (arrows). D. 4 months later bone-to-bone appearance with complete obliteration of joint space at the medial compartment is observed (arrows).
-

Figure 2
Figure 2. Subchondral insufficiency fracture (SIF) and spontaneous osteonecrosis of the knee (SPONK). A. Anterior-posterior X-ray shows medial femoral surface (arrow). B. Radiograph of advanced SIF with deformity of the medial femoral surface (arrow). Note marked subchondral sclerosis reflecting structural remodeling in the subchondral bone due to altered local biomechanical loading.
-

Figure 3
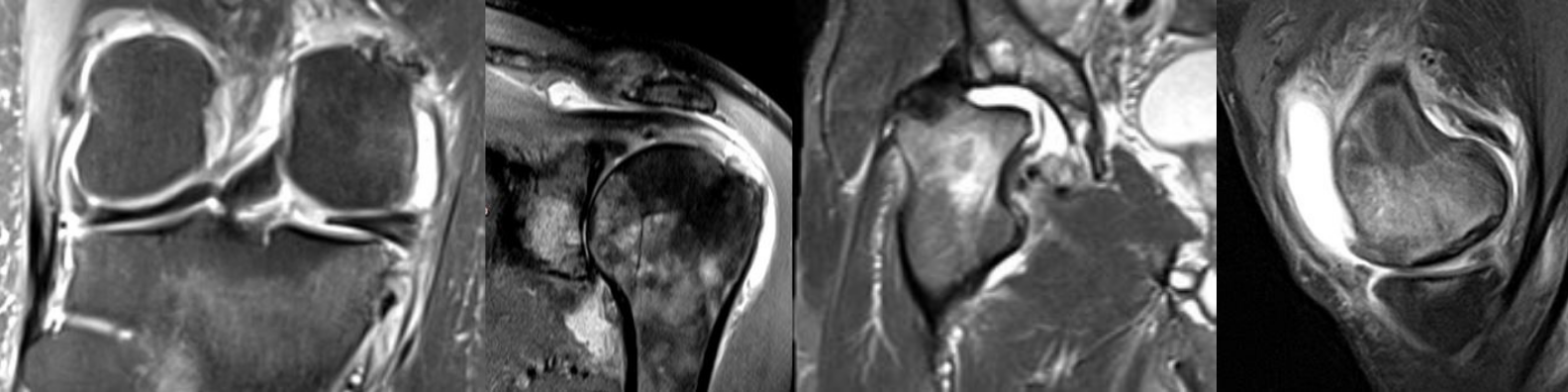
Figure 3. Osteonecrosis. A. Coronal proton density-weighted MR image shows areas of osteonecrosis in the medial femur (thick arrows) and in the metaphyseal tibia (thin arrows). These are usually incidental findings on MRI. Note characteristic serpiginous hyperintense demarcation and central areas of fat equivalent signal. B. SIF with necrosis and unfavorable outcome. A. Proton density-weighted fat suppressed image depicts edema-like signal as diffuse hyperintensity (large arrows). In addition there is a focal area of hypointensity directly adjacent to the subchondral plate representing subchondral necrosis (small arrow). Articular surface is at risk for collapse. C. The same knee (coronal proton density weighted mage) 9 months later shows progression of subchondral necrosis (arrows), collapse of the articular surface and marked surrounding diffuse bone marrow edema-like alterations.